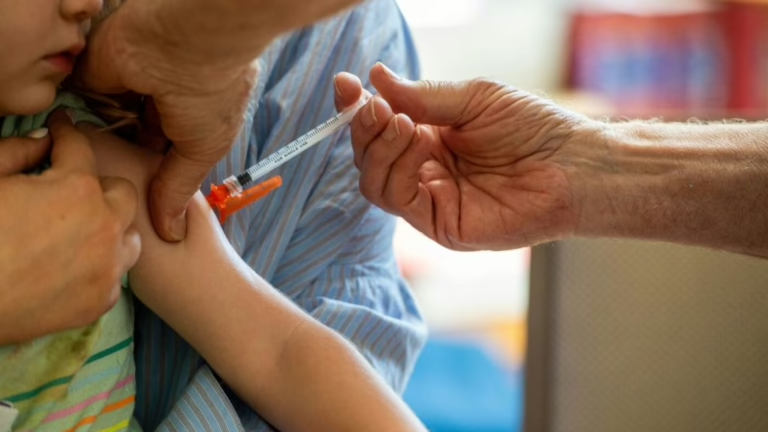
Many individuals have found it challenging to obtain a COVID-19 vaccine recently, with confusion persisting due to the CDC’s pending guidance on vaccine administration.
Joseph Prezioso/AFP/Getty Images
toggle caption
Joseph Prezioso/AFP/Getty Images
Remy Sweeney-Garrett is eager to have her daughters vaccinated against COVID-19, yet she has encountered significant obstacles in doing so.
“I feel anxious and exasperated,” shares Sweeney-Garrett, a 34-year-old Seattle resident raising her 9-year-old daughter Maxine and toddler Maeve, who is 18 months old. “Honestly, it’s infuriating.”
The primary barrier is the absence of finalized CDC guidelines for vaccine distribution, which are essential for the federal Vaccines for Children Program to begin supplying doses to healthcare providers and public health agencies. This program covers roughly half of the children in the United States who qualify for vaccination.
“My youngest is particularly vulnerable to respiratory illnesses, and the thought of her ending up hospitalized terrifies me,” Sweeney-Garrett explains. “It’s maddening because this situation seems entirely manageable if the government acted promptly.”
Such a delay from the CDC is highly atypical. Usually, the agency moves swiftly-sometimes within hours-to issue directives, especially given the urgency of immunizing the population ahead of the winter respiratory virus season.
Dr. Susan Kansagra, chief medical officer at the Association of State and Territorial Health Officials, notes, “This postponement has sown confusion not only among families but also among healthcare providers uncertain about the current status.”
Sweeney-Garrett’s predicament is shared by many.
“Parents inquire daily about vaccine availability. They want their children protected,” says Dr. Elias Kass, the family’s pediatrician. “Yet, we have no vaccines in stock, no timeline for delivery, and no clear answers. We have the tools to prevent illness, but as a society, we’re missing the mark.”
The CDC’s hesitation has also complicated vaccine access for adults, who face a patchwork of inconsistent state regulations. Despite efforts in some regions to simplify the process, others still require prescriptions, and some pharmacies turn away individuals unable to prove eligibility under new criteria.
Vaccine policy expert Dorit Reiss from the University of California, San Francisco, describes the situation as “a preventable chaos.” She warns, “The decision to delay action will likely lead to increased COVID-19 cases and greater public health consequences.”
The Department of Health and Human Services, which supervises the CDC, has not provided comments regarding the delay.
This year’s COVID vaccine rollout has been notably disorganized. Previously, anyone six months or older could receive vaccines at pharmacies without prescriptions. However, the FDA’s recent approval limits eligibility to individuals at higher risk due to age or health conditions, sparking widespread uncertainty.
Compounding the issue, the CDC’s Advisory Committee on Immunization Practices-recently influenced by Health Secretary Robert F. Kennedy Jr., who appointed members skeptical of vaccines-has introduced additional procedural requirements. While these recommendations could broaden eligibility and expand pharmacist involvement, the CDC has yet to endorse them, leaving distribution plans stalled.
“This bottleneck is a major obstacle to improving vaccine access,” Kansagra emphasizes. “It’s a significant setback.”
With the federal government currently shut down, the timeline for CDC action remains unclear, heightening concerns as the winter respiratory season approaches.
Dr. Philip Huang, director of Dallas Health and Human Services, expresses apprehension: “The severity of this season is unpredictable but could be severe. It’s disheartening that our efforts to protect the community are being hindered.”