On Monday, October 13, the World Health Organization (WHO) released a critical medical product alert concerning three cough and cold syrups manufactured in India. The agency urged health authorities worldwide to report any sightings of these medicines in their markets and to intensify focused monitoring efforts, particularly within informal distribution networks.
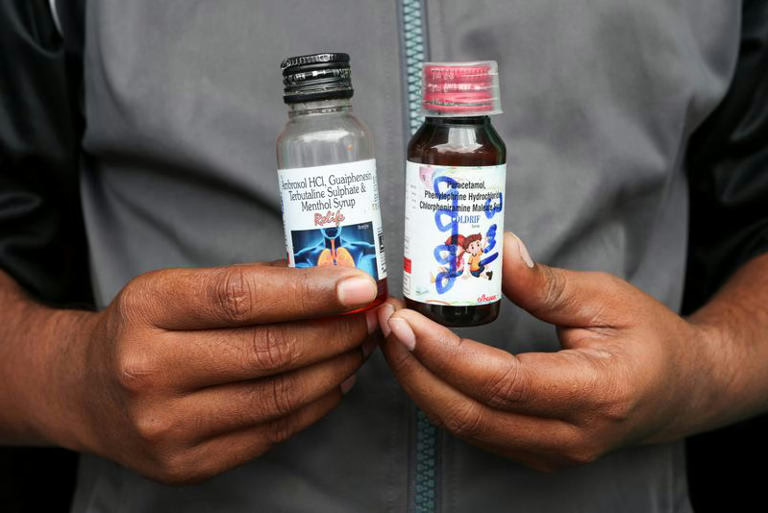
The syrups in question include certain batches of Coldrif by Sresan Pharmaceutical, Respifresh TR from Rednex Pharmaceuticals, and ReLife produced by Shape Pharma. According to India’s Central Drugs Standard Control Organization (CDSCO), laboratory analyses conducted on October 8, 2025, revealed the presence of diethylene glycol (DEG)-a hazardous chemical-in at least three oral liquid formulations. Alarmingly, the concentration of this toxic solvent was found to be nearly 500 times above the safe threshold. These contaminated syrups were reportedly administered to children under five years old in Chhindwara, Madhya Pradesh, who subsequently succumbed to fatal outcomes.
WHO’s statement highlights that these oral liquid medicines contain active compounds typically used to alleviate symptoms associated with the common cold, influenza, or cough. The contaminated products pose grave health dangers, potentially causing severe and life-threatening conditions. Diethylene glycol is known to be poisonous to humans when ingested and can result in death. The agency categorizes these syrups as substandard since they do not comply with established quality criteria and specifications, warning that their use-especially in young children-could lead to serious harm or fatality.
In response, CDSCO has directed state authorities to cease production at the implicated manufacturing facilities, revoke product licenses, and initiate recalls of the affected batches. The regulator confirmed to WHO that these contaminated medicines have not been exported outside India, with no evidence indicating illicit international distribution. Nevertheless, WHO recommends that national regulatory bodies conduct targeted market surveillance, focusing on all oral liquid medicines produced at the same sites since December 2024.
The WHO alert details the potential toxic effects of DEG ingestion, which include severe abdominal pain, persistent vomiting, diarrhea, difficulty urinating, headaches, confusion, and acute kidney failure-conditions that can be fatal if untreated. The organization stresses the urgency of identifying and removing these hazardous products from circulation and encourages healthcare professionals to report any detections or adverse reactions through their national pharmacovigilance systems.
For the general public, WHO’s guidance is clear: anyone possessing these syrups should refrain from using them. If you or someone you know has consumed these products or experienced unexpected side effects, seek immediate medical attention or contact a poison control center. The agency also reminds consumers to purchase all medical products exclusively from authorized and licensed suppliers. Individuals with information regarding the manufacture or distribution of these syrups are urged to contact WHO at [email protected].



















0 Comments